In addition, the instrument aspirates MALDI matrix solution (CHCA in ACN/H2O/TFA) as well as a solution of two known peptides for optional internal calibration, and it dispenses them to the MALDI target before or after the transfer of the re-dissolved peptide solutions. These solutions are provided in 0.5 mL Sarstedt or Eppendorf tubes. Of course, the instrument could dispense other solutions as well, and the samples must not be provided in a dried state, etc.
Off-line nano- or micro-LC-MALDI-MS and -MS/MS workflow:
This application is for the performance of proteomics research and to identify and characterise complex peptide mixtures (shot gun proteomics workflow).
The instrument is used as a non-contact fraction collector and starts doing this on demand, controlled by a TTL or shortcut signal sent by the LC software. The fraction collection works the following way:
The effluent of the LC is combined with a dispensing solution, and they jointly send off as a series of little droplets (on-demand, time-controlled) down to the MALDI target. Each droplet can be dispensed to a different position on that target (one droplet, one fraction), or several (user's choice) can be collected on the same position to enhance the detection sensitivity and maintain the chromatographic resolution.
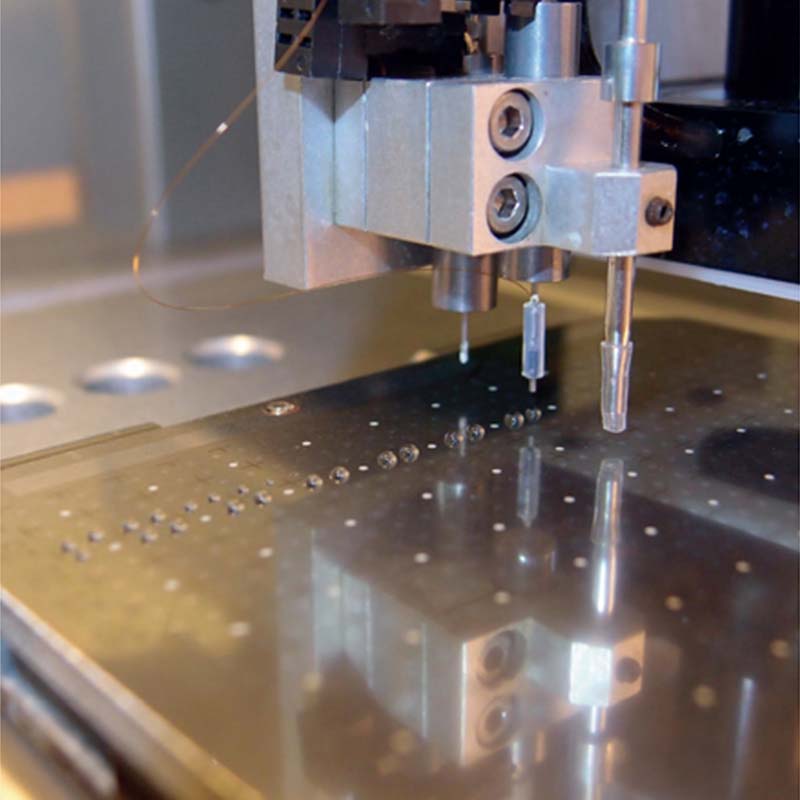
In this case, it is a fused silica capillary with an outer diameter of 90 um and an ID of 20 um coming from, for example, a Dionex nano-HPLC in a special T-connector. It enters our dispensing tip with a dispense orifice (channel) with an ID of 200 um. That tip is filled with dispensing solution (e.g. 20% ACN/80% H2O).
Each time a droplet of the dispensing solution with a typical volume of 50 nL (or larger, e.g. 100, 200 or 500 nL) is sent off, that amount passes the end of the capillary and travels along the eluted peptides, which have accumulated in the form of a tiny droplet. The accumulation time is set by the user, e.g. 1, 2, 3, 10 or 20 seconds. The time frame depends on the LC flow. The small size of the droplets ensures that the sample is not too diluted.
The non-contact operation is what separates our instruments from our competitors’. It minimises sample carry-over and excludes contamination of the tip by target contacts (dust particles etc.), damage of the capillary or failure to deposit a fraction caused by variations in height (non-perfectly planar targets).
Other tasks achieved with the use of the same instrument are the dispensing of the matrix solution, with or without internal standards or an acidic water (pH < 2) for the washing/conditioning of thin-layer sample preparations. To make this possible, the instrument also provides a sucking nozzle (capillary) that works much like a vacuum cleaner: it sucks the washing solution (sitting droplet of 1-3 uL) away without touching the target (non-contact removal).
All in all, the instrument provides two dispense channels (LC effluent + one other solution) and one liquid removal channel, which can be combined in any way.